What Is the Electron Pair Geometry for S in Sof4
Where the electron pair is in the structure is important because every molecule assumes a particular structure because it is lowest in energy it conserves the most energy and is more stable relative to other conformations. K 4 Ge 4 Cs18-crown-6 2 e Group 2 Elements.
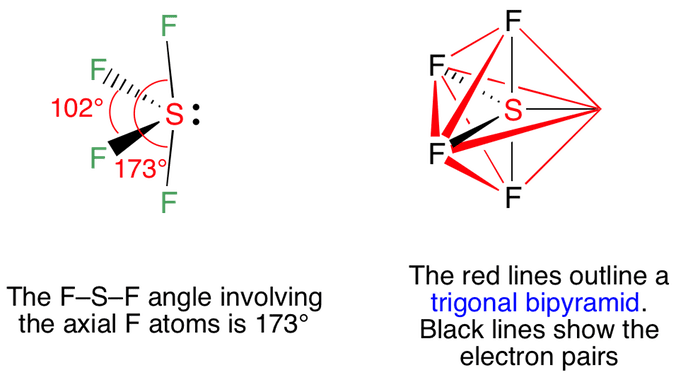
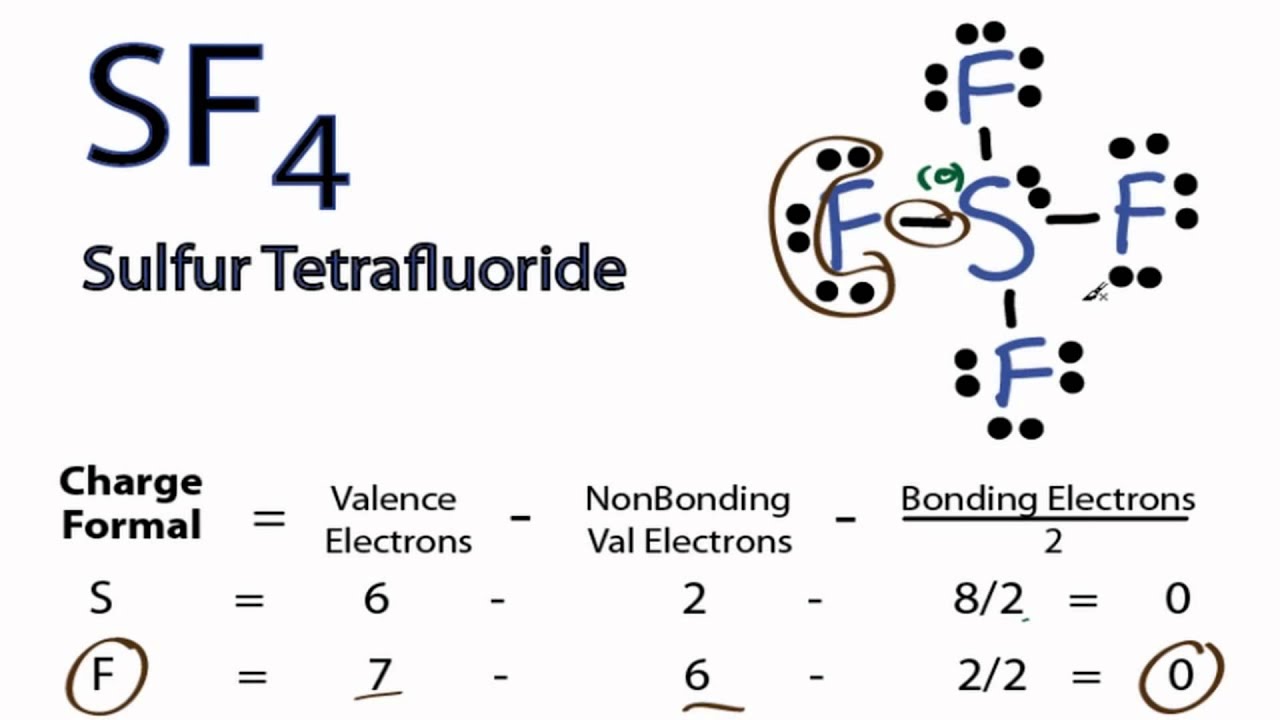
Vsepr Sf4 Sulfur Tetrafluoride
What is the electron-pair geometry for S in SOF4.
. Click and drag the molecle to rotate it. There are lone pairs around the central atom so the molecular geometry shape of BrFg is B. 84Predict the electron pair geometry and the molecular structure of each of the following ions.
The drawing of NH2Cl lewiss structure is simple and easy to understand Just follow the steps we have written for you. There is one lone pair present on the central atom of the NH2Cl lewis structure. Second determine the 3D molecular geometry with VSEPR rules.
A Cl-F bond is a dipole since F is more electronegative. The molecular geometry of the AsO2- ion is bent because of the lone electron pair with the central arsenic atom making the O-As-O bond. There are lone pairs around the central atom so.
_____ There are. Li 2 NH antifluorite LiNH 2 defect antifluorite Group 1 Elements. What is the electron-pair geometry for S in SOF4.
34 valence electrons. Valence electrons of Sulfur. What is the electron-pair geometry for Al in AlF3.
What is the electron-pair geometry for Br in BrF5. ____ There are ____ lone pair s around the central atom so the molecular geometry shape of SOF4 is ____ B. The geometry is square pyramidal and is due to 6 electrons pairs around the central chlorine atom one of which is nonbonding.
The Lewis structure Lewis structure is a pictorial representation of bonds and valence electrons in the molecule. 22 Related Question Answers Found. Jmol_Canvas2D Jmol jmolApplet0 x.
There are a total of thirty-four valence electrons. 7x4 valence electrons Total. Caesium Peroxide Cs 2 O 2.
Valence electrons of Fluorine. B Is SOF4 in the same point group. Use solid and dashed to show 3D geometry if needed.
S has 6 valence electrons plus 1 for each S-F single bond and 2 for the SO. Experts are tested by Chegg as specialists in their subject area. 6 valence electrons Fluorine.
Solution for FES O bond angle in SOF4. There are lone pairs around the central atom so the molecular geometry shape of SOF4 is This problem has been solved. Submit Answer 5 question attempts remaining.
The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur in which there are four bonding pairs and one lone pair. We review their content and use your feedback to keep the quality high. This electron arrangement is known as Trigonal Bipyramidal.
Total 10 electrons five pairs. Follow some steps for drawing the lewis dot structure for NH2Cl. The shape is like a seesaw.
Calcium Carbonate CaCO 3 Polymorphs. The chlorine atom in the molecule CIF3 has 5 valence electron pairs out of which 3 are bonding pairs and 2 are loan pairs so the molecule has a trigonal bipyramidal electron pair geometry and T shaped molecular geometry due to lp-lp and lp-bp repulsion. What is the electron-pair geometry for S in SOF4.
The SF4 Lewis structure is the combination of 34 valence electron and 5 electron pairs around the Sulfur where there are 4 bonding pairs and 1 lone pair. Dipotassium Pentasulfide K 2 S 5 Lithium nitride Li 3 N Na 172 In 192 Pt 2. In SF4 the four fluorine atoms have three pairs of electrons in the octet which uses twenty-four valence electrons.
First draw the lewis structure. Draw lewis structure sof4. Solution for Draw the Lewis for structure for SOF4 including all the lone pair electrons.
Chemistry questions and answers. These valence electrons are for covalent bonding. SOF4 - Sulfur Monoxide Tetrafluoride.
In the formation of SF4 the sulfur atom compound produces bonds with the fluorine where there are eight valence electrons. 100 5 ratings Aelectron pair geometry of S in SOF4 is Trigonalbi. What is the electron-pair geometry for S in SOF4.
Within the context of VSEPR theory you can count electrons to determine the electron geometry parent geometry. There are lone pairs around the central atom so the molecular geometry shape of Bel2 is A. The See-sawstructure where the electron pair occupies an equitorial position is a lower-energy structure than trigonal pyramidalelectron.
4 7 as there are four fluorine atoms we have to consider valence electrons of all atoms Total number of valence electrons in SF4 number of valence electrons in sulfur number of valence electrons in fluorine. Structure trigonal bipyramid with double bond equatorialclass AX 5. 34 valence electrons You can put sulfur in the middle because fluorine tends to make single bonds.
We review their content and use your feedback to keep the quality high. Experts are tested by Chegg as specialists in their subject area. Point group C 2v.
Count total valence electron in NH2Cl. View the full answer. What is the electron-pair geometry for Cl in ClF5.
6 28. Therefore you can put 6x4 on each fluorine 2x4 to account for four single bonds and 2. Therefore SOF4 is polar.
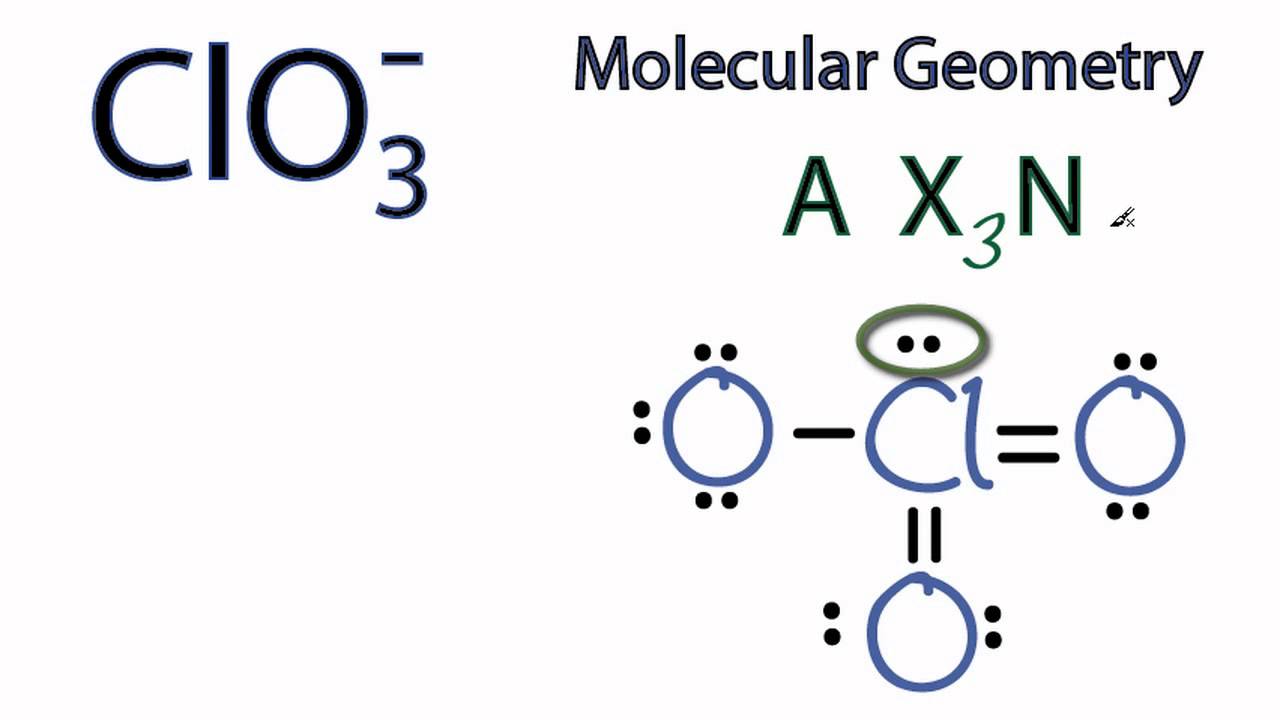
Clo3 Molecular Geometry Shape And Bond Angles Chlorate Ion Youtube

Sulfur Tetrafluoride Oxide F4os Pubchem
10 3 Vsepr Geometry Chemistry Libretexts

Sef4 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

Sf4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Electronic Geometry Of Sf4 Quora

Predicting The Shape Of The Sf4 Molecule Youtube

Sf4 Molecular Geometry Shape Youtube
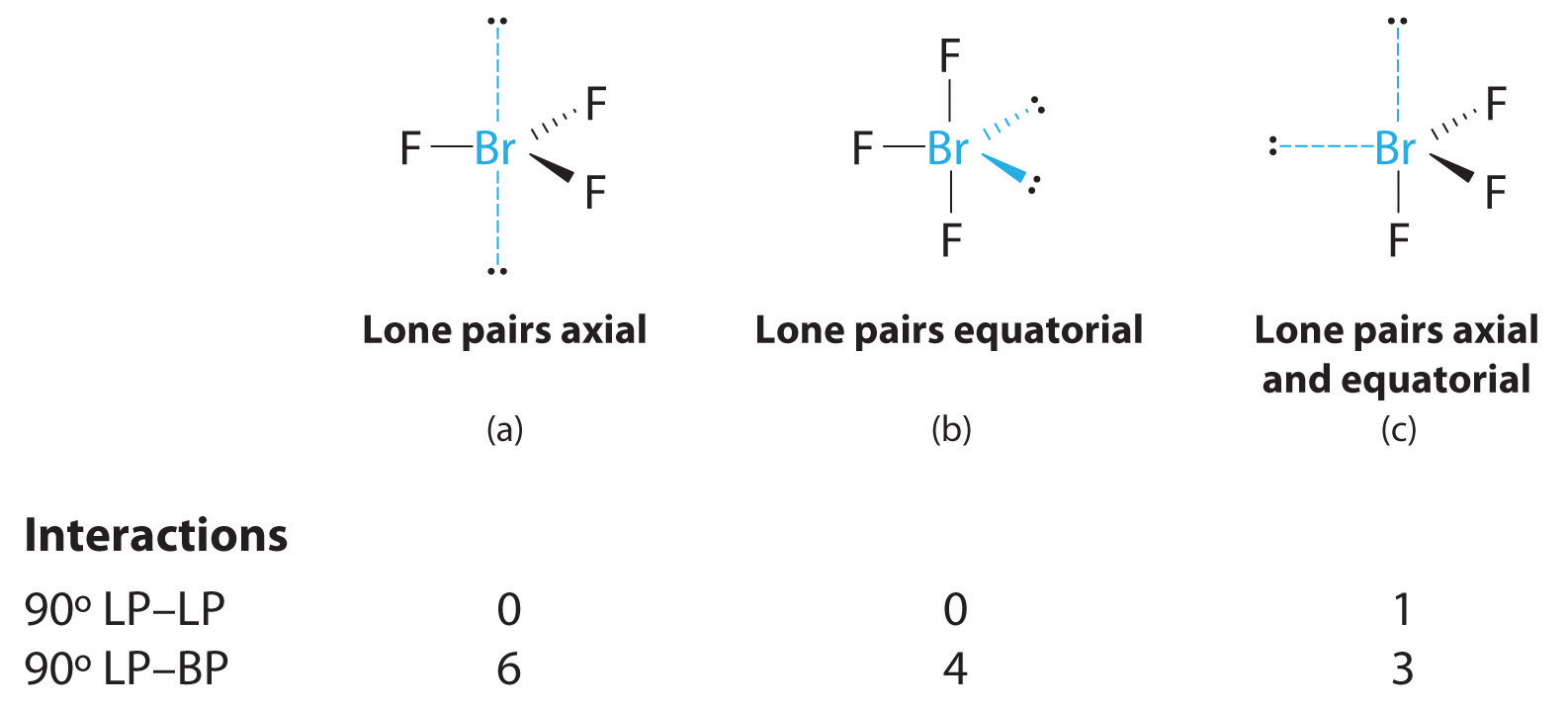
10 3 Vsepr Geometry Chemistry Libretexts

Sif4 Molecular Geometry Bond Angles Electron Geometry Youtube
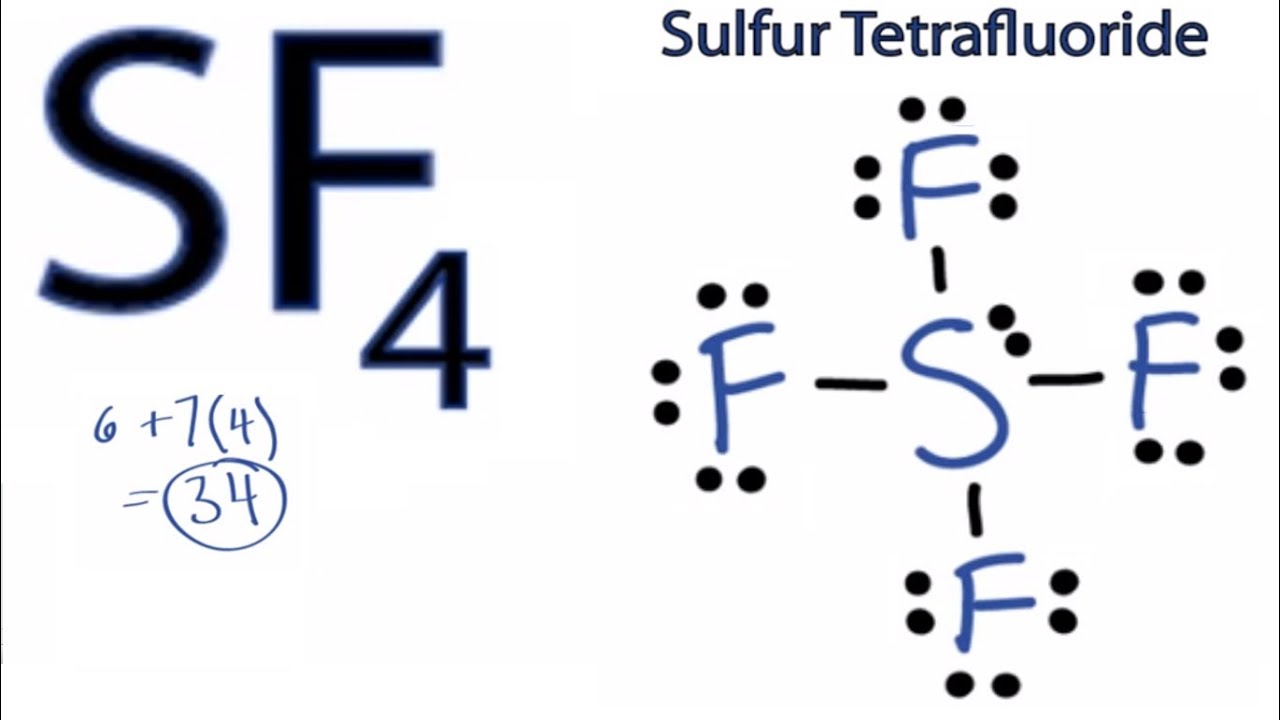
Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube
What Is The Molecular Shape Of Sof4 Quora

What Is The Molecular Geometry Of Sf4 Quora

Sbr2 Molecular Geometry Shape And Bond Angles Molecular Geometry Molecular Geometry
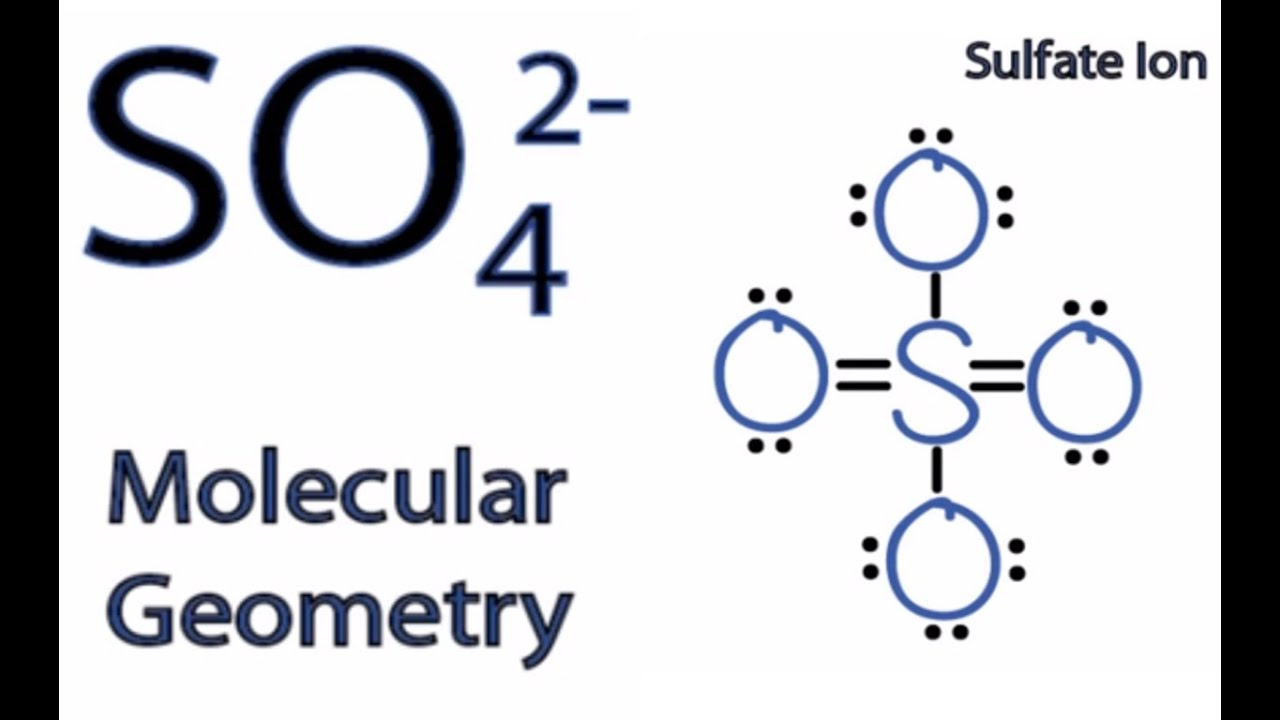
So4 2 Molecular Geometry Shape And Bond Angles Youtube

Sef4 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
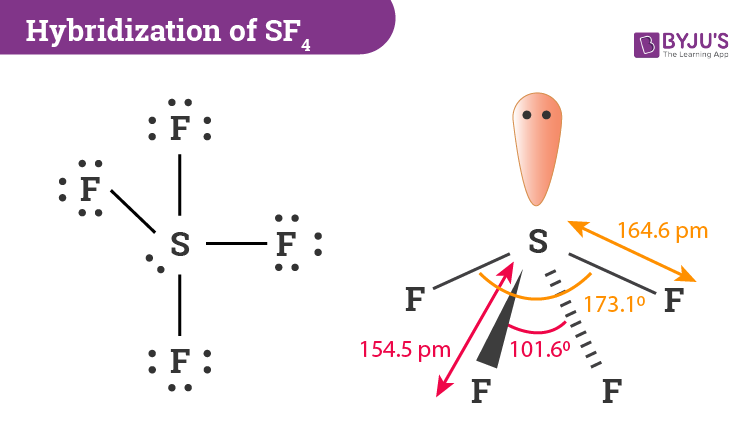
Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride
This information is very helpful, Thank You for sharing such valuable information with us.Best school bangalore
ReplyDelete